BEHIND CTKCLIP
Enter the USA Market with Your OTC Products
Your One-Stop Solution for OTC Product Entry into the U.S.
Can’t find what you are looking for? Drop us an ‘Inquiry’ and we will reach out shortly!
InquiryBEHIND CTKCLIP
Enter the USA Market with Your OTC Products
Your One-Stop Solution for OTC Product Entry into the U.S.

Unlock the Potential of Your OTC Product Line
When it comes to creating Over-the-Counter (OTC) products, choosing the right partner is the key to success. At CTKCLIP, we help you to bring your vision to life. From formulation to launch, we ensure your products are safety, effective, and ready to stand out in the market.
These everyday essentials—like sunscreens, acne treatments, and skin protectants—are a staple in consumers’ daily routines. Unlike regular cosmetics, OTC products are classified as medications, which means they must meet strict regulatory standards. Navigating these rules can feel overwhelming, but that’s where we come in. With our expertise, we’ll guide you through the complex world of OTC product development, making the journey smoother and your success inevitable.

Fast-Track Product Development
Creating safe and effective OTC products requires innovative formulas and strict adherence to regulatory standards. With CTKCLIP’s ready-to-go formulas, you can launch your product 6 to 10 months faster than the general development timelines. Proudly made in the USA, our solutions ensure that your product meets all necessary regulations while accelerating your time to market.
Start Small, Grow Big: Optimized MOQ Solutions
Launching a new product can feel overwhelming, especially when manufacturers demand large order quantities. At CTKCLIP, we understand the importance of flexibility. That’s why we offer consulting for lower quantities, providing the optimal MOQ solutions for each product. Whether you're new brand or introducing niche products, our approach minimizes financial risk, making it easier to get started and giving your brand the freedom to grow without the heavy commitment.

Reliable Manufacturing You Can Count On
When it comes to product quality and safety, reliability is everything. In an industry where compliance is not just important but essential, you need a partner who prioritizes both. With FDA-certified, U.S.-based facilities that adhere to the highest pharmaceutical standards, we ensure every product is made with care and precision, meeting even the strictest inspection criteria.
The Importance of Safety and Compliance
When it comes to your products, safety is our top priority. We ensure that every active ingredient in our formulations is carefully evaluated for both safety and effectiveness. We share our OTC development expertise and guide you through the process to ensure your product meets U.S. market standards and complies with regulations.
Explore OTC Product Possibilities
Whether you’re looking to develop a sunscreen, an acne treatment, or a skin protectant, CTKCLIP has the expertise to guide you through the process. We specialize in these categories and can help you create products that are not only compliant but also stand out in the crowded market.;
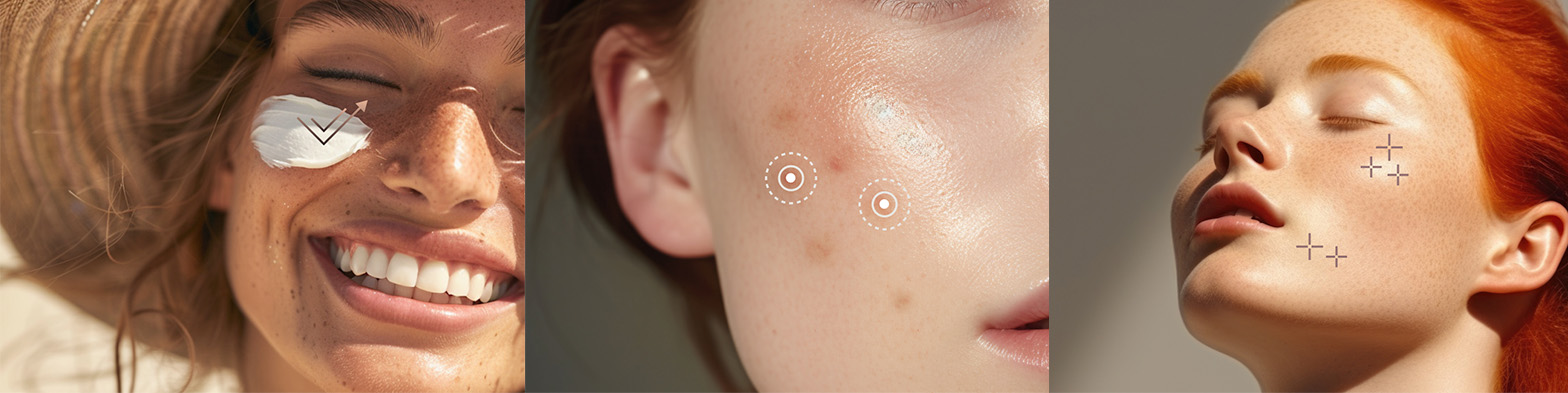
Let’s Make Your OTC Product a Reality
Ready to take the next step in developing your OTC product? Leave us an inquiry, and our team of experts at CTKCLIP will guide you through every step of the process. We’re here to help turn your vision into a product that consumers trust and love. Reach out today, and let’s create something extraordinary together!
BEHIND CTKCLIP
17
JAN
25

BEHIND CTKCLIP
17 JAN 2025

BEHIND CTKCLIP
27 DEC 2024

BEHIND CTKCLIP
04 DEC 2024

BEHIND CTKCLIP
14 NOV 2024

TRENDS&INNOVATION
07 NOV 2024
Target all stakeholders involved in the company's unethical behavior. However, matters related to slander that are not based on facts and personal privacy that are not related to work will not be included.
- Deceptive advertising, infringement of intellectual property rights, violation of personal information protection
- Corruption, such as workplace harassment, violation of gender equality, embezzlement of company assets, leakage of company intellectual property rights, or unfair use
- Illegal acceptance of money and valuables or unfair acts against partners, collusion with competitors, and fraudulent solicitation of stakeholders
- Manipulation of documents and coefficients, contrary to the interests of shareholders and the company
- Violations of environmental and human rights protection, and win-win violations with local communities
- Other violations of ethical management
The informant's personal information and the contents of the report are protected, and no information is disclosed or implied without the informant's consent.
- You can report the report anonymously.
- However, in the case of anonymous reports, if the content is not specific or the facts are unclear, the investigation may not proceed.
- Violations of informant protection, such as retaliation against informants or providing disadvantages, are strictly punished in accordance with in-house regulations such as employment rules.
- The reporting section is protected by a secure security system, and the reporting investigation is handled under security by a designated person
Collection information: Name and contact information
Purpose of collection: Processing complaints according to reports and replying to the results of processing
Retention period: It is held until the above purpose is achieved, and is not used for any purpose other than confirmation of information.